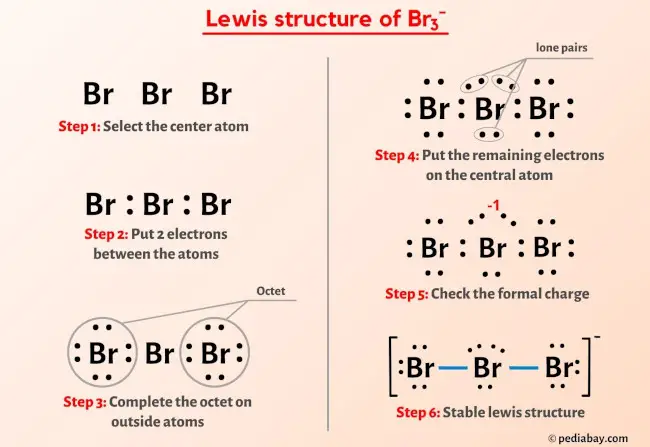
While I3- and Br3- are both stable molecules, F3- is not a stable molecule. Provide an explanation. | Homework.Study.com

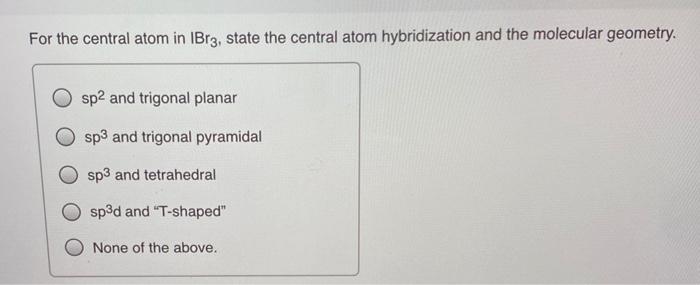
SOLVED: Question 9 (10 points) What is the hybridization of the central atom in AsBr32- (OR AsBr3 2-)? sp sp² sp³ sp³d sp³d² Question 10 (10 points) What is the hybridization of
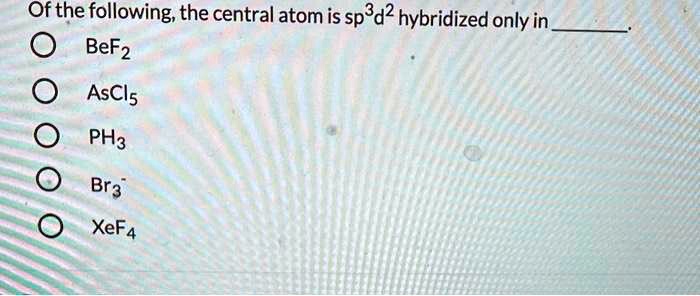
SOLVED: Of the following, the central atom is sp3d2 hybridized only in BeF2, AsCl3, PH3, Br3, O, and XeF4.
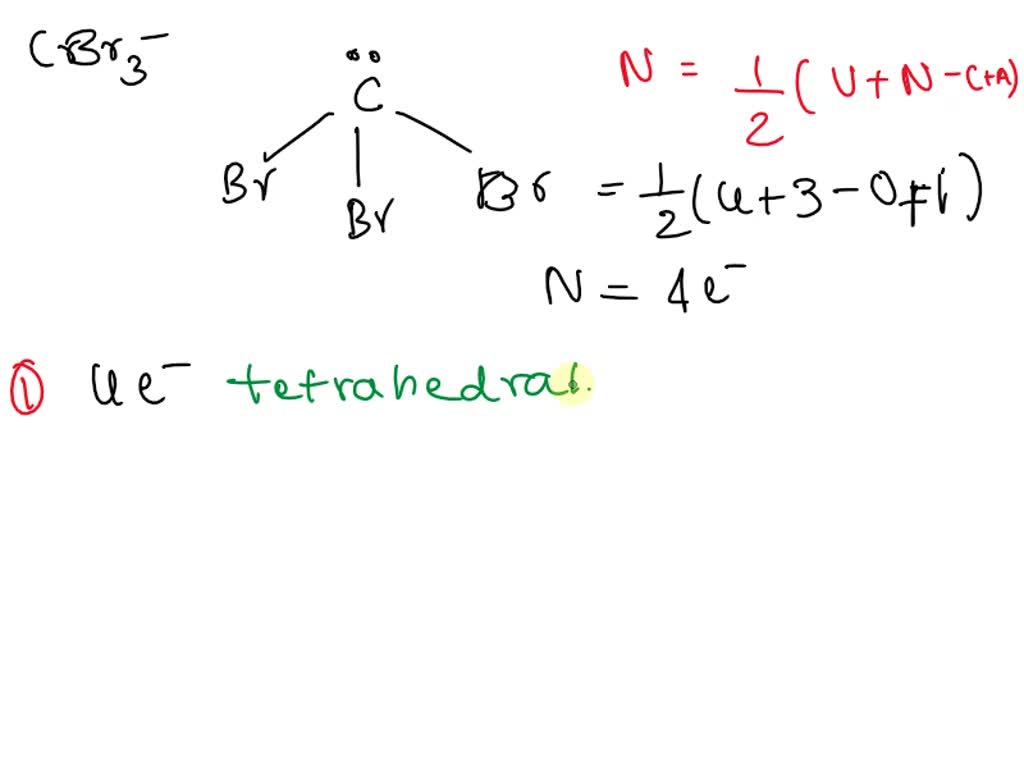
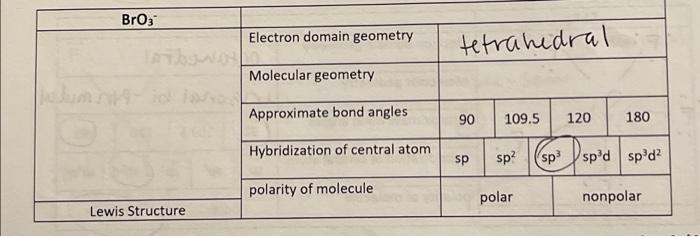
SOLVED: Give the electron geometry, molecular geometry, and hybridization for CBr3-. Electron geometry: trigonal planar Molecular geometry: trigonal pyramidal Hybridization: sp3

SOLVED: Based on hybridization, what is the polarity of Br3? A) Non-polar B) Ionic C) It cannot be determined. D) Polar Based on hybridization, what is the polarity of Brs? A) Non-polar