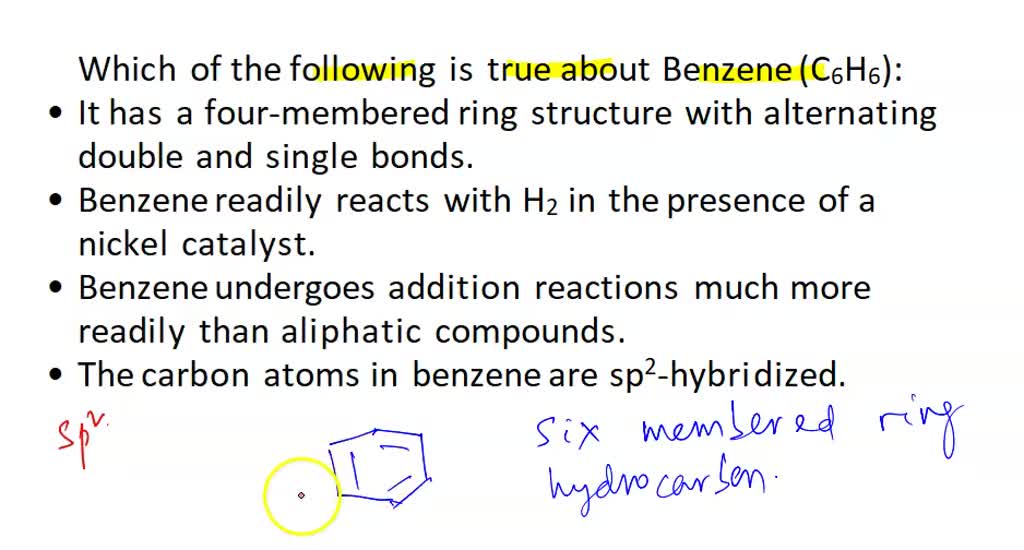
SOLVED: Which of the following is true about Benzene (C6H6)? It has a six-membered ring structure with alternating double and single bonds. Benzene readily reacts with H2 in the presence of a
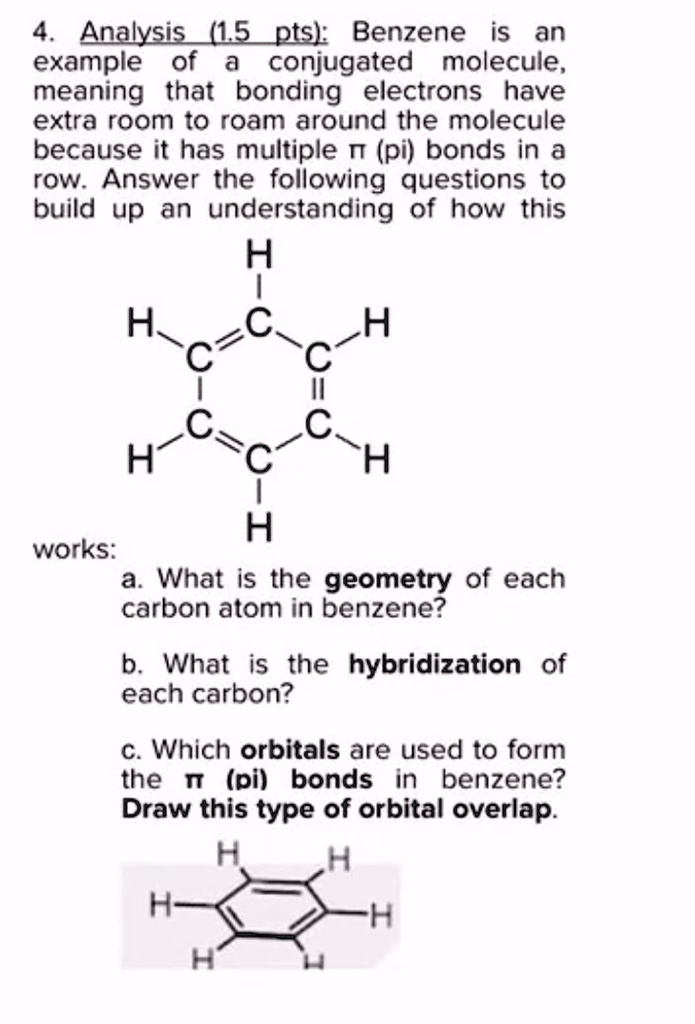
SOLVED: Benzene is an example of a conjugated molecule, meaning that bonding electrons have extra room to roam around the molecule because it has multiple π (pi) bonds. In order to understand

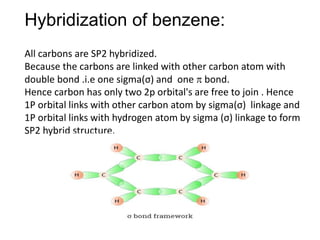
OneClass: In benzene what is the hybridization of each carbon atom?(please refer to image) In benzene...

C6H6 lewis structure, molecular geometry, bond angle, hybridization | Molecular geometry, Molecular, Molecular shapes
Why does benzene form sp2 hybridisation and not sp3? Why does one zth 2p orbital not participate in hybridization? Can someone explain this briefly? - Quora
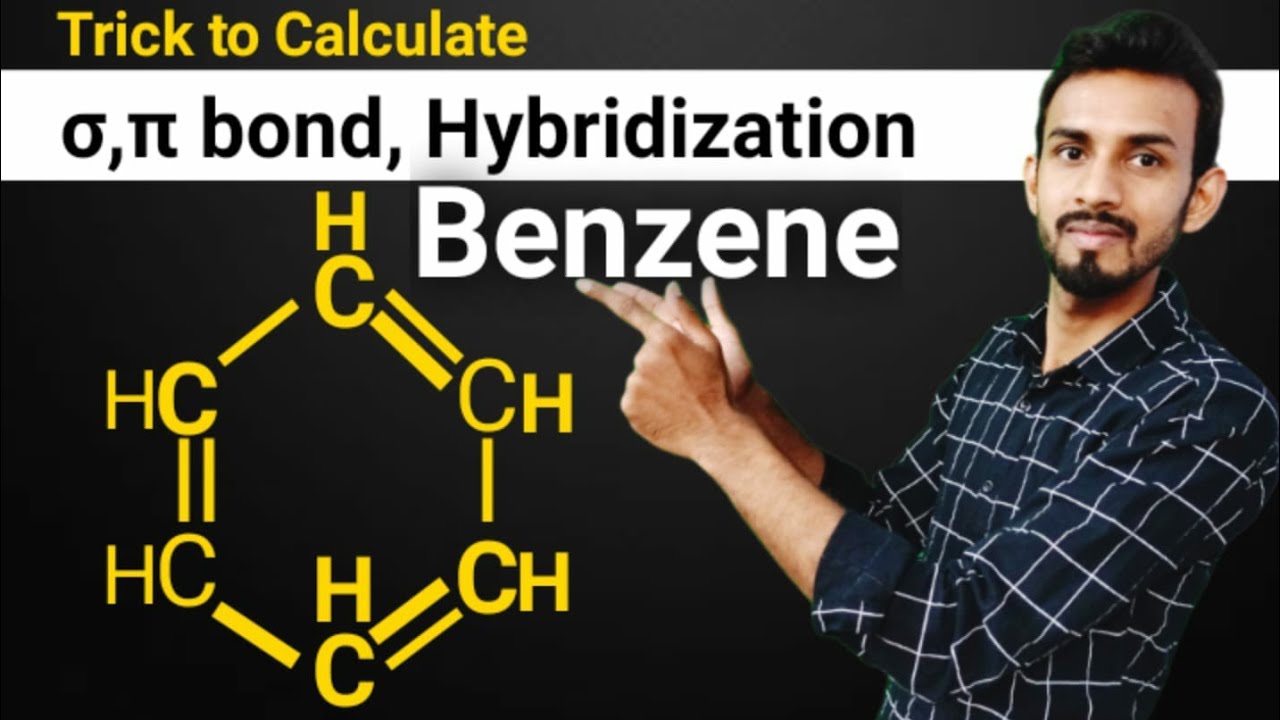
Trick to find hybridization of carbons in benzene | How to find sigma and pi bonds in benzene - YouTube

Benzene Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram - Techiescientist