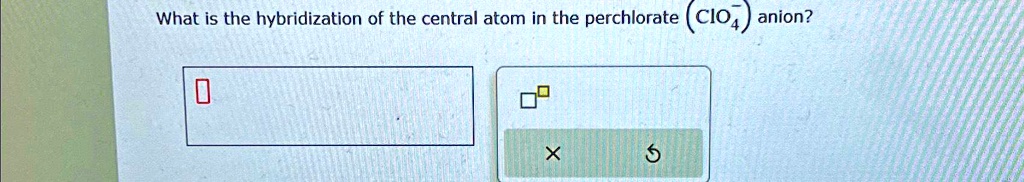
SOLVED: What is the hybridization of the central atom in the perchlorate ( ClO(4)^(-))anion? ◻◻ What is the hybridization of the central atom in the perchlorate (CIO4) anion? 5

How many of the following have Cl atom in sp^3 hybridised state?ClO_2, Cl_2O, Cl_2O_7, Cl_2O_6, ClO^-_45432

Hybridization of Chlorine in Hypochlorite ClO-, Chlorite ClO2-, Chlorate ClO3-, Perchlorate ClO4- - YouTube

Hybridization of Chlorine in Hypochlorite ClO-, Chlorite ClO2-, Chlorate ClO3-, Perchlorate ClO4- - YouTube

The hybridization of Cl atom in ClO_(4)^(-) and ClO_(3)^(-) is | CLASS 12 | NONE | CHEMISTRY | D... - YouTube

The type of hybrid orbitals used by the chlorine atom in \( \mathrm{ClO}_{2}^{-} \)is: (A) \( s ... - YouTube

Hybridization of Chlorine in Hypochlorite ClO-, Chlorite ClO2-, Chlorate ClO3-, Perchlorate ClO4- - YouTube

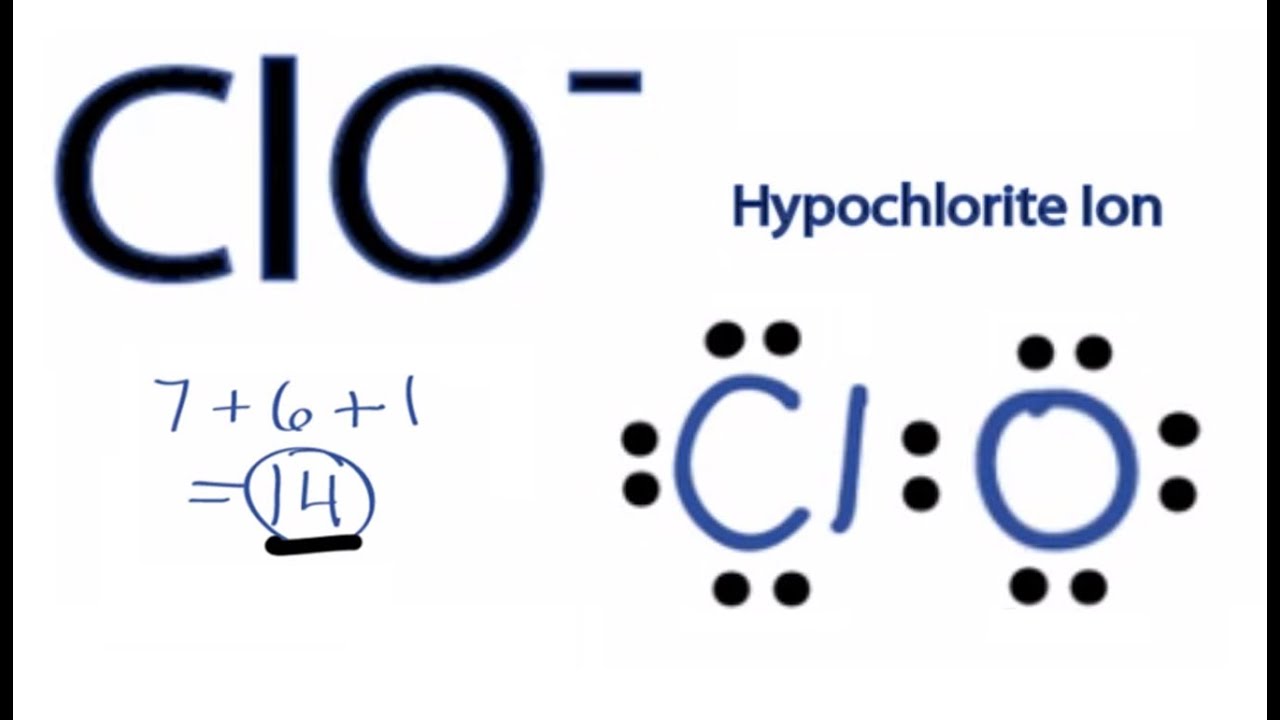
What is the steric number of ClO- ? Specify and draw ClO- electron pair geometry. What is ClO- hybridiztion | Homework.Study.com

Qtion no 7​​ 7 Correct statement(s) for ClO4- is (A) Total number of electron taking part - Chemistry - Solutions - 12713323 | Meritnation.com