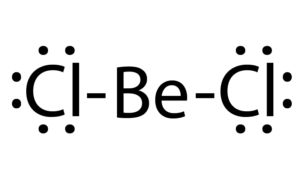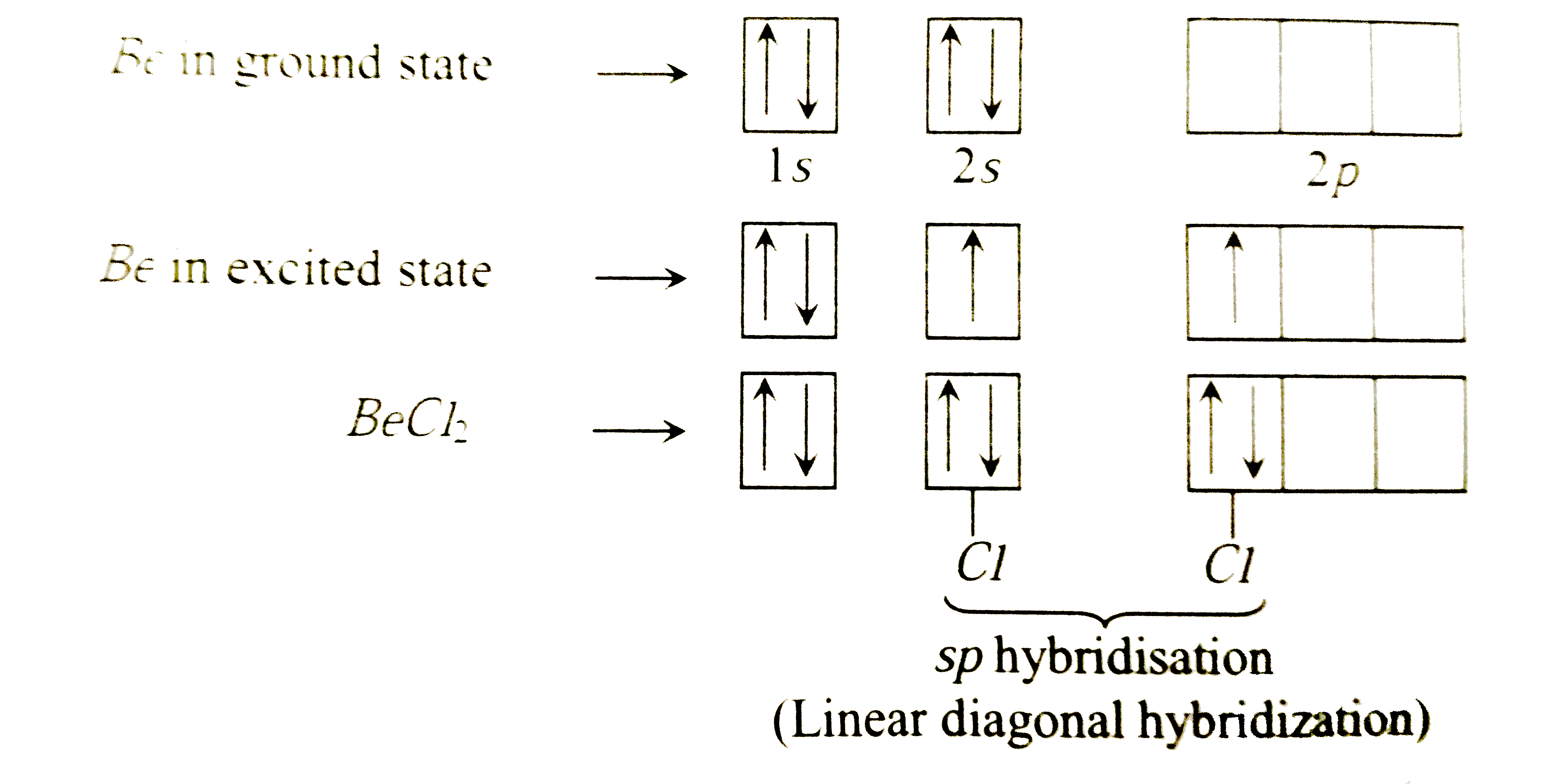what is Hybridisation? Based on Hybordisationwrite the formation of given below molecules?1) Becl2 2)NH3 3) - Brainly.in
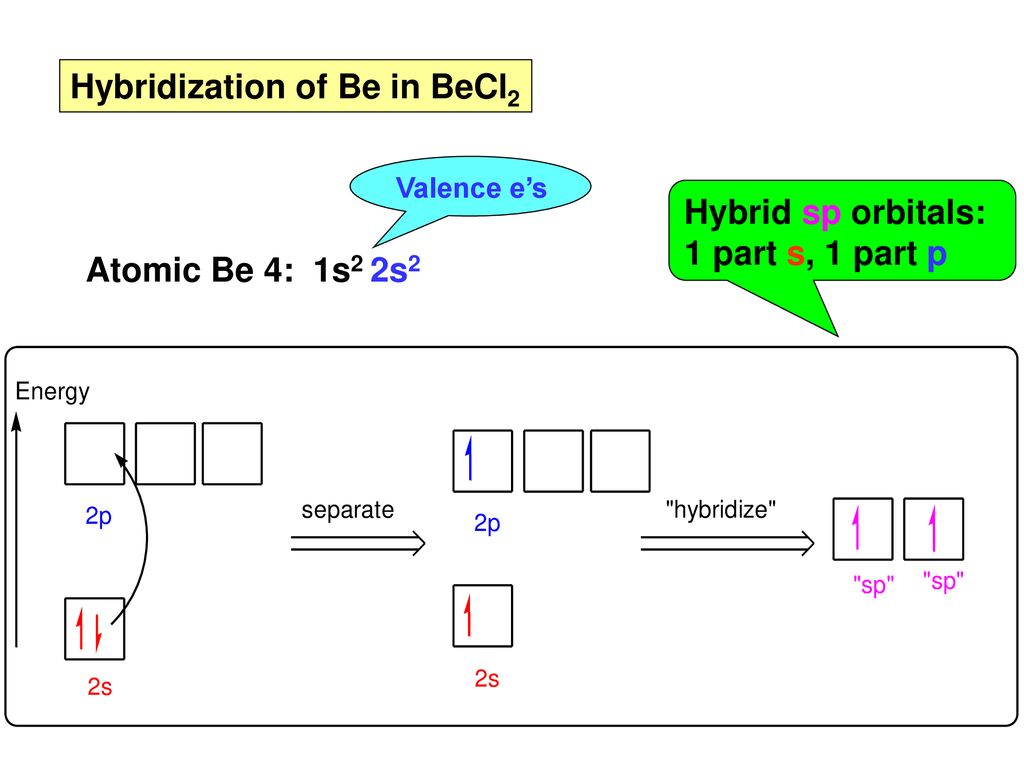
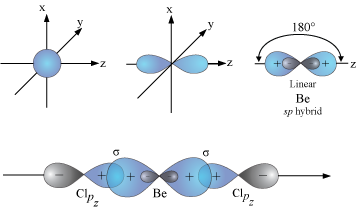
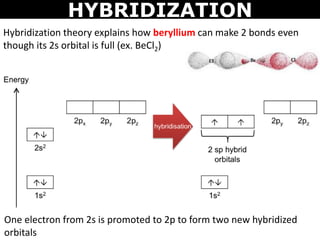
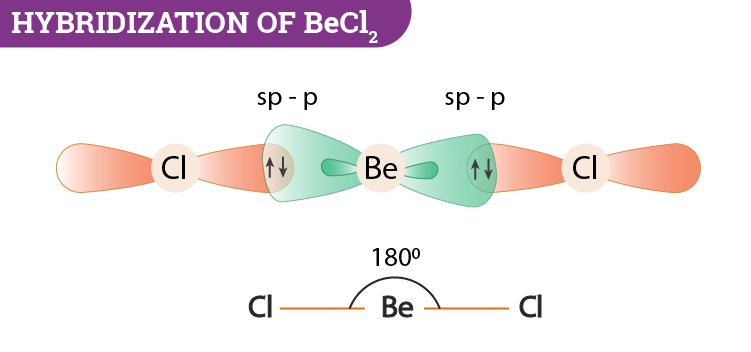
When does the Hybridization occur In becl2 there are 2 electron in 2s orbital of be then what - Chemistry - Electrochemistry - 13733947 | Meritnation.com

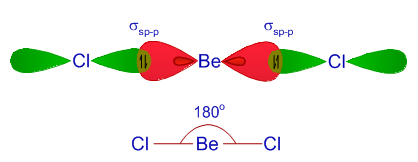
BeCl2 Lewis structure, Molecular geometry, Hybridization, Bond angle and shape - Geometry of Molecules
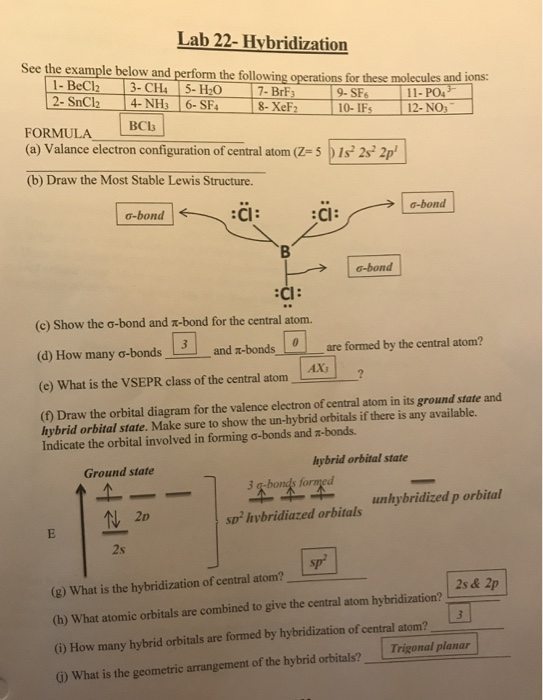
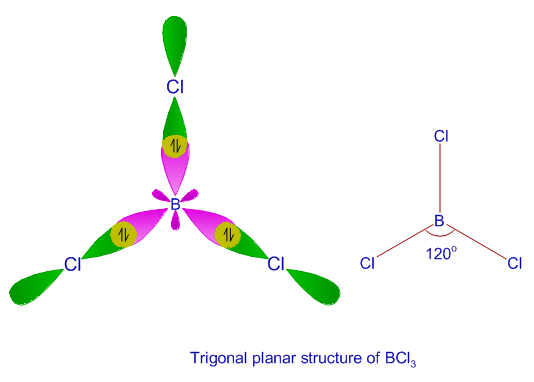
Exemplars of Hybridization in Chemistry - askIITians Blog - One place for all updates on IIT JEE & Medical Exams

BeCl_{2} can exist in monomeric form, dimeric form as well as polymeric form. Identify the hybridisation of Be in polymeric BeCl_{2}spsp^{2}sp^{3}sp^{3}d
How to calculate the hybridization state in the given formula of compund. Ex: The hybridization of BeCl2 in solid state and above 1200K is respectively (1) sp3, sp3 (2) sp3, sp2 (3)

BeCl2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram - Techiescientist