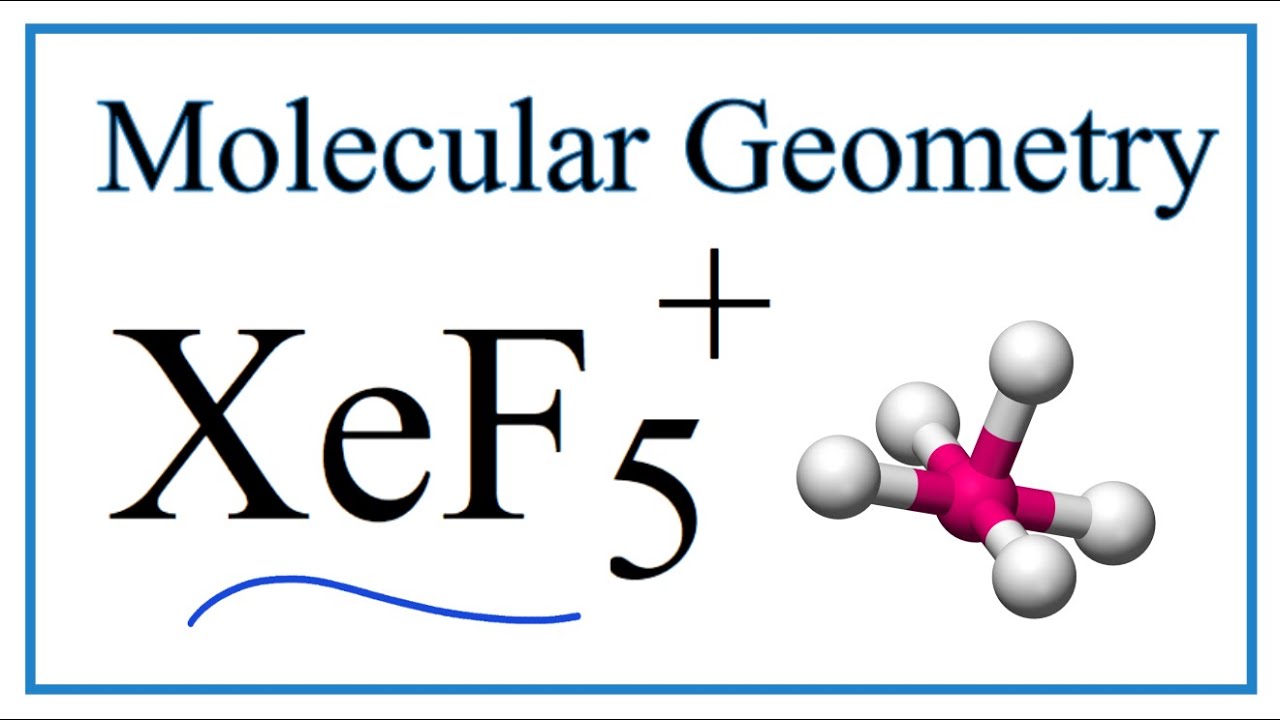
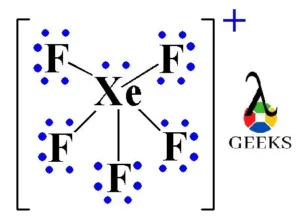
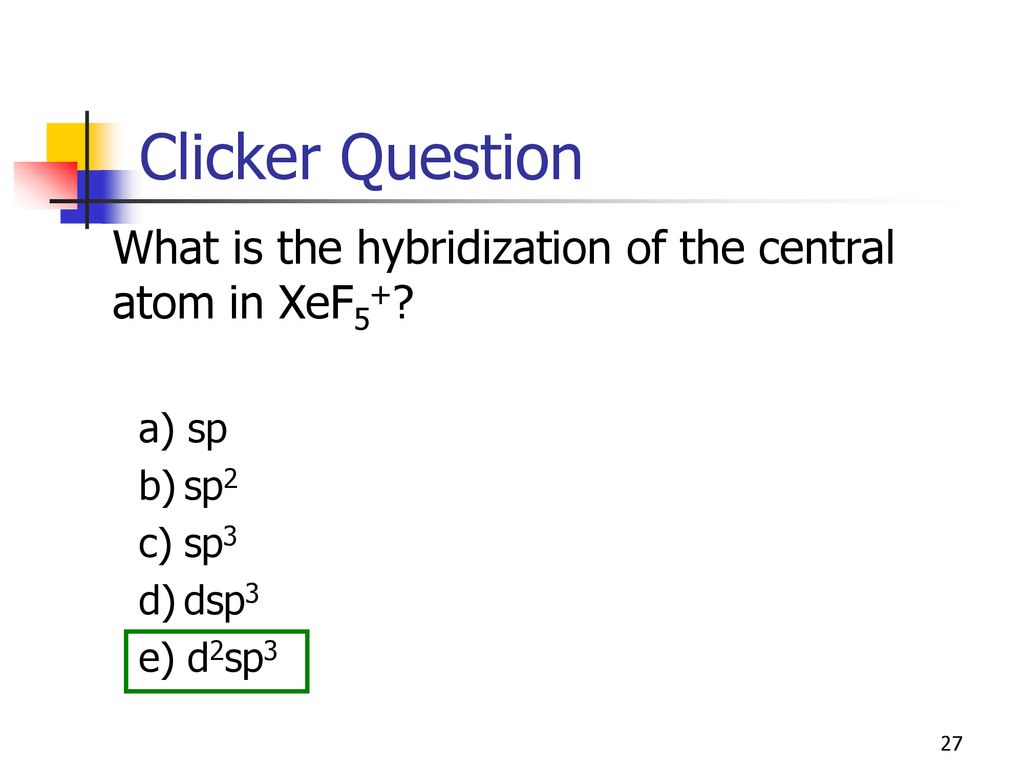
What is the steric number and molecular geometry of XeF_5^+? Are there any distortions due to loan pairs? | Homework.Study.com
Why does Xenon Hexafluoride exist as [XeF₅]⁺ [F]¯ and not [XeF₅]⁺ [XeF₂]¯ in the solid state? - Quora
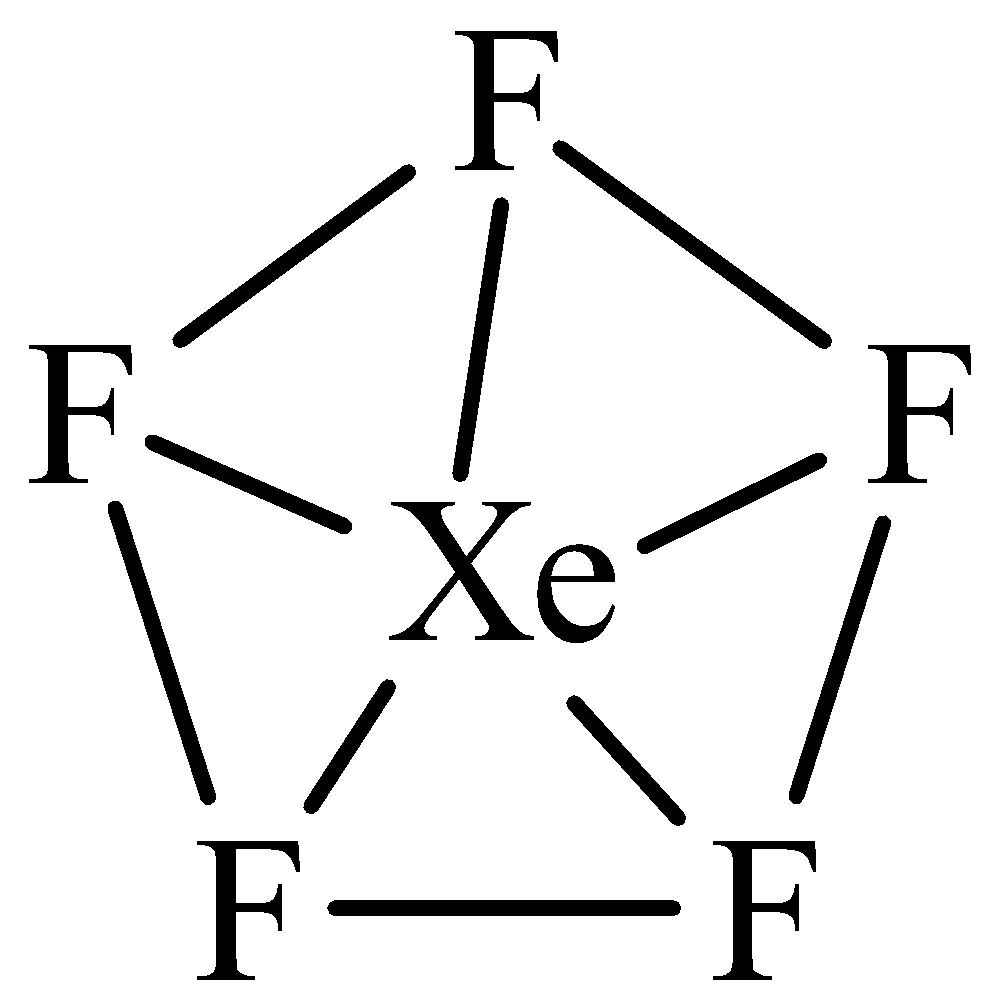
The hybridization and shape of $Xe{F_5}^ - $ is.A) $s{p^3}$, tetrahedron.B) $s{p^3}$, trigonal pyramidal.C) $s{p^3}{d^2}$, trigonal bipyramidal.D) $s{p^3}{d^3}$, pentagonal planar.

XeF5+ Lewis Structure: How to Draw the Lewis Structure for XeF5+ (Xenon pentafluoride cation) - YouTube
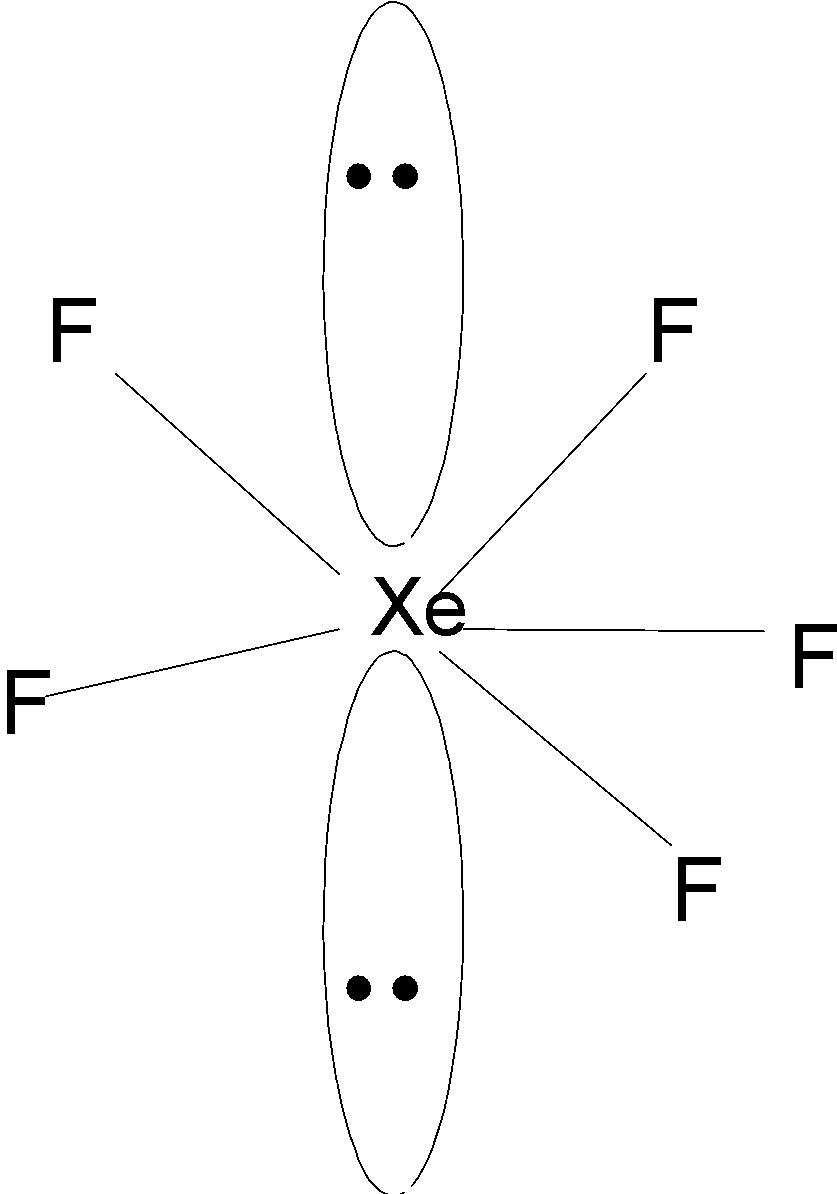
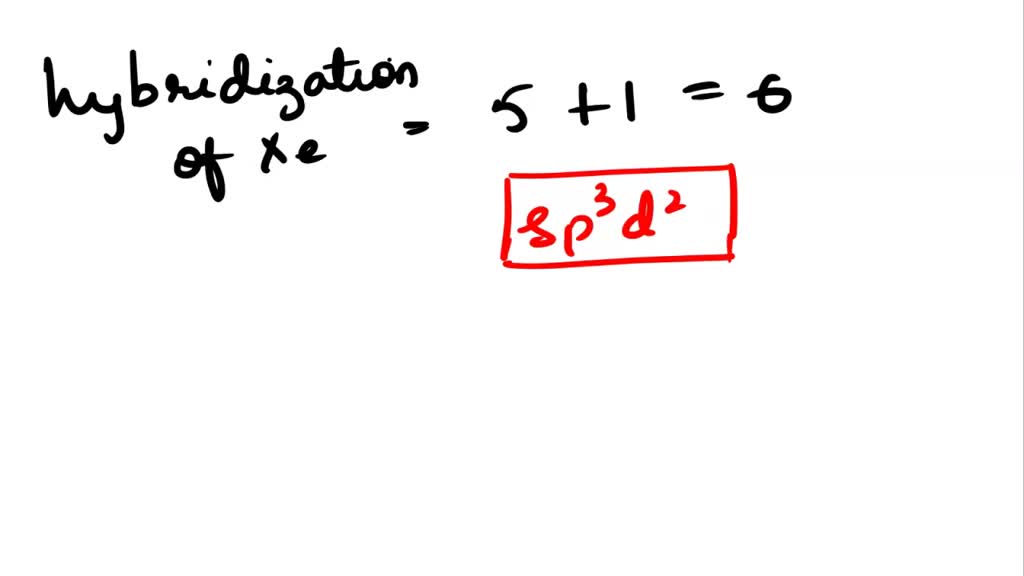

![The shape/structure of [XeF5]– and XeO3F2, respectively, are: The shape/structure of [XeF5]– and XeO3F2, respectively, are:](https://byjus-answer-creation.s3.amazonaws.com/uploads/8399Chemistry_62b6e470fe666010d3ec1df0b.jpg_img_upload_solution_2022-07-28%2012:54:00.369186.png)
![The shape/structure of [XeF5]– and XeO3F2, respectively, are: The shape/structure of [XeF5]– and XeO3F2, respectively, are:](https://byjus-answer-creation.s3.amazonaws.com/uploads/8399Chemistry_62b6e470fe666010d3ec1df0.jpg_img_upload_solution_2022-07-28%2012:54:16.579050.png)